Under Construction 
Contents: Characteristics | Description | Uses | Sources (Refining, Fermentation, Biomass) | Terms | Links
This page started out as a fuel page, but the scope has expanded to include solvents and things like alcohols used in food and drinks.
Characteristics:
| Type | Chemical | Boiling Point Range °C | BTU/lb
(1,000) | Use |
| Hydrogen | H | -253 | 52-61 | Fuel Cells or Gasoline alternative. |
Gaseous hydrocarbons
LPG | C1 Methane (Natural Gas)
C2 Ethane (C2H6)
C3 Propane (C3H8),
C4 Butane (n-butane, isobutane)(C4H10),
C5 Pentane | 0-20 | 20.9-23.7
21.6
19.9-21.6.
19.5-21.2.
20.1- | Gas fuels for cooking and heating.
|
| Alcohol | C1 Methanol (CH3OH),
C2 Ethanol (C2H5OH)
C3 Isopropyl (C3H7OH) | 20-100 | 0.91.
12.8.
14 | Solvents for varnishes, dry cleaning
Disinfectants |
Naphtha _
(Stoddard Solvent) | C5 Pentanes
C6 Hexanes [Aromatics, Toluene(C7), Benzene(C6), Xylene(c6)]
C7 Heptane | 27-221 | 19.3-20.7 | Solvents for varnishes, dry cleaning, Car wax |
| Gasoline | C5 Pentane, C6 Hexane, C8 Octane, C9-C12 | 30-205 * | 18.7-20 | Fuels for internal combustion engines |
| Kerosene | C10 Decane (mostly), C12-C18 | 175-300 | | Jet engine fuel and diesel fuel #1 |
| Diesel, Heating Oil, Waxes | C16 cetane (Hexeadecane), C18 - C24 | 300-400 | 18.4-19.7 | Diesel fuel #2 |
Cn - n Carbon atoms in each molecule.
See: Heating Value and density of Hydrogen and Fuels
and Gas Composition Table with Heating values
* Gasoline is formulated differently for varying climate conditions. As weather gets colder manufacturers bring the initial boiling point down to help cold engines start easier.
MJ/l - megajoules per liter - Another measure Energy Content
To convert from Btu/lb you need the density of each fuel.
Mass, Weight, Density or Specific Gravity of Liquids
Description:
- Diesel
 - #1 Diesel (Diesel 1-D, Kerosene-like, kerosene) - So similar to kerosene that many manufactures make a dual purpose product that is sold as both kerosene and diesel #1. This fuel can be ultra low, low, or high sulfur. It has better solvent qualities than #2.
- #1 Diesel (Diesel 1-D, Kerosene-like, kerosene) - So similar to kerosene that many manufactures make a dual purpose product that is sold as both kerosene and diesel #1. This fuel can be ultra low, low, or high sulfur. It has better solvent qualities than #2.
A typical molecule would be cetane, or n-hexadecane (C16H34)
- #2 Diesel (Diesel 2-D, Home heating oil, No. 2 Gas/Burner oil) - The most common grade, which specifies a fuel that is suitable for most cars and trucks. It is preferred because it has a higher energy content than grade 1-D.
However, it does not burn as cleanly or smoothly as a grade 1-D fuel, which is now used in many city busses to reduce smoke in the exhaust and to reduce the odor of diesel exhaust.
Winter diesel fuel is #2 mixed with #1 for lower temperature gel point.
- Kerosene - Decane (called Paraffin in the U.K.)
 Gasoline Gasoline
Gasoline contains over 500 alkanes, cycloalkanes and other hydrocarbons with 3 to 12 carbons.
A typical breakdown of modern gasoline (excluding additives and oxygenated compounds) might be 15% n-paraffins (examples: Pentane, Hexane, Heptane, octane, decane, etc.); 30% iso-paraffins (examples: 1-methylpropane, 2-methylbutane, 2,2,3-Trimethylbenzene, etc.); 12% cycloparaffins (example: cyclohexane, cyclopentane, etc.); 35% aromatics (examples: Benzene, Toluene, ethyl benzene, 1,3,5-trimethylbenzene; m-Xylene, etc.); and 8% olefins (examples: 2-pentene, 2-methylbutene, cyclopentene, etc.). The octane number of the gasoline is a function of the components.
The boiling range from 86 to 428°F (30 to 220°C)
See: Octane
- Unleaded Gasoline - Auto gasoline is more volatile than white gas, produces noxious combustion products, and will clog the stove quicker than about anything else. Extremely explosive, is very caustic, releases a terrible odor that lingers, and its additives and high octane components release deadly vapors when burned. It may also prematurely clog your stove when compared to white gas.
- White Gas - Super unleaded gas.
(a mix of heptane, octane, and nonane, plus some aromatics , that means ring-type hydrocarbons like benzene, um, what is called benzene in the US, not what is called benzene in Germany. marketed as lamp and stove fuel
- Environmental gasoline - This should be "free" from aromatic hydrocarbons and olefins such as benzene and many of the toxic additives in regular unleaded gasoline for your auto.
In many places today you will see camp stove (e.g. Coleman) fuel and white gasoline used synonymously. They are not the same. Coleman Fuel was developed in the early 50's as a replacement for "white gas". Coleman fuel contains components (such as naphtha) that are much less volatile than gasoline .
 Camp Stove fuel (Coleman Fuel, Calumet Lantern Fuel) - contains components that are much less volatile than gasoline (such as naphtha). Coleman fuel contains about 50% naphtha, 50% aliphatic petroleum distillates, 2% xylene, 2% toluene, 0.5% benzene, green dye and rust inhibitors. Camp Stove fuel (Coleman Fuel, Calumet Lantern Fuel) - contains components that are much less volatile than gasoline (such as naphtha). Coleman fuel contains about 50% naphtha, 50% aliphatic petroleum distillates, 2% xylene, 2% toluene, 0.5% benzene, green dye and rust inhibitors.
Similar products:
Charcoal lighter fluid - Some of these are a mix of kerosene and naphtha and some are just straight naphtha.
Petroleum Spirits
 Butane, n-butane - Works well at high altitudes but burns poorly below 40° F due to poor vaporization at low temperatures. Butane, n-butane - Works well at high altitudes but burns poorly below 40° F due to poor vaporization at low temperatures.
Butane is commonly used in backpacking and portable stoves, lighters, small torches and as a propellant for aerosol cans.
Boiling point: 31° F (0.5°C). Fuel will not vaporize well below its boiling point.
Butane - Wikipedia
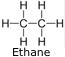 Ethane (C2H6) - Chemicals in the ethyl group are formed by replacing a hydrogen atoms in ethane with another functional group.
Ethane (C2H6) - Chemicals in the ethyl group are formed by replacing a hydrogen atoms in ethane with another functional group.
Its chief use is as feedstock for ethylene production.
See ethylene in household chemicals.
-
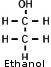 Alcohol Alcohol
- Ethyl alcohol (C2H5OH) (Ethanol)
. Denatured - Ethanol to which a poisonous (denaturing) substance, such as acetone or methanol, has been added to make it unfit for consumption.
Available in paint departments.
. Grain Alcohol (aka pure ethanol, pure grain alcohol (PGA), grain neutral
spirits (GNS), rectified spirit, rectified alcohol, medical grade
ethanol, ethyl anhydrous, moonshine, over-proof rum) is non-toxic and can double for medicinal uses.
. Lab grade ethanol may have benzene or other chemicals mixed in with it.
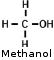 - Methyl Alcohol (CH3OH) (Methanol, Wood Alcohol, methyl hydrate, liquid
fondue fuel, some camp stove fuel)- The Automotive fuel line de-icer Heet is pure methanol. - Methyl Alcohol (CH3OH) (Methanol, Wood Alcohol, methyl hydrate, liquid
fondue fuel, some camp stove fuel)- The Automotive fuel line de-icer Heet is pure methanol.
Ethanol auto fuel - Flex-fuel Vehicles (FFV) are designed to run on gasoline or gasoline-ethanol blends of up to 85% ethanol (E85).
See Flex-fuel Vehicles | FuelEconomy.gov and Alternative Fuels Data Center: E85: An Alternative Fuel | energy.gov
- Rubbing (71% or 91% Isopropyl [2-propanol] or Ethyl) alcohol to which some methyl alcohol and/or acetone has been added to make it poisonous. It may contain water also.
Isopropyl alcohol (C3H7OH) produces soot when burned in simple stoves, because the extra carbon atom creates a mixture which is too rich.
Other:
ethylene glycol [C2H4(OH)2] (Antifreeze),
glycerine [C3H5(OH)3] used to make plastics, drugs, foods, cosmetics and nitroglycerine.
See: Alcohols at ScienceByJones
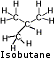 Isobutane - Isobutane is a structural isomer of butane with a lower boiling point. Manufactures claim that isobutane provides a steady flow without tapering off as the canister empties and is added to butane to increase its performance. Because of the greater vapor pressures of isobutane compared to butane, you may experience much greater flow that may blow itself out if turned up too high or possibly even damage a stove not designed for isobutane. Isobutane - Isobutane is a structural isomer of butane with a lower boiling point. Manufactures claim that isobutane provides a steady flow without tapering off as the canister empties and is added to butane to increase its performance. Because of the greater vapor pressures of isobutane compared to butane, you may experience much greater flow that may blow itself out if turned up too high or possibly even damage a stove not designed for isobutane.
Boiling point: 11° F (-12°C). Fuel will not vaporizes well below its boiling point.
Butane - Wikipedia
- Isopropane - The term isopropane suggests that this is structural isomer of propane, but since propane is a three carbon hydrocarbon, a structural isomer doesn't exist. Isopropane is instead a commercial term used to describe isobutane/propane mixes (and sometimes butane/propane mixes).
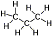 Propane (a type of LPG) - Great hot burning fuel that works at low temperatures and at high altitudes. Due to high pressures of propane (close to five times that of butane at room temperature), propane canisters are made of thick heavy steel. Pure propane is not recommended for stoves designed to run mostly butane and/or isobutane without a pressure regulator. Propane (a type of LPG) - Great hot burning fuel that works at low temperatures and at high altitudes. Due to high pressures of propane (close to five times that of butane at room temperature), propane canisters are made of thick heavy steel. Pure propane is not recommended for stoves designed to run mostly butane and/or isobutane without a pressure regulator.
Propane is generally used for heavy duty stoves, lanterns, heaters and torches.
Boiling point: -43° F (-40°C). Fuel will not vaporizes well below its boiling point.
 LPG mixes (20-40% Propane) (Butane/Propane, Butane/Isobutane/Propane, Isobutane/Propane, IsoPro, IsoPropane) -
The higher the propane content the longer it will burn at lower temperatures. You may still end up with most of the butane and/or isobutane remaining in the canister if using a stove below 31° F (0.5°C) or 11° F (-12°C) respectively. LPG mixes (20-40% Propane) (Butane/Propane, Butane/Isobutane/Propane, Isobutane/Propane, IsoPro, IsoPropane) -
The higher the propane content the longer it will burn at lower temperatures. You may still end up with most of the butane and/or isobutane remaining in the canister if using a stove below 31° F (0.5°C) or 11° F (-12°C) respectively.
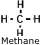 Natural Gas - A mixture of gaseous hydrocarbons, primarily methane.
Also contains ethane, butane, propane. Natural Gas - A mixture of gaseous hydrocarbons, primarily methane.
Also contains ethane, butane, propane.
Also referred to as Compressed Natural Gas (CNG) and Liquefied Natural Gas (LNG)
In addition to the above ethylene, butylene, isobutane, and isobutylene can be retrieved from the natural gas liquid.
Source: Primarily from underground reservoirs like oil, but also is made from biomass.
Uses: Primarily used for home heating, but also used for generation of electricity and motor-vehicle fuel.
Accounts for 23% of US Energy Consumption
Dimethyl Ether (DME) -- A synthetic diesel fuel derived from natural gas, is an excellent candidate for direct-injection engines. DME contains no sulfur and burns more cleanly.
See also: Common Household product chemical composition
Uses:
Home Heating
Camp Stoves
Motor-vehicles:
Standard:
Gasoline
Diesel
Alternative:
Propane
Gasohol (10% Ethanol Blend)
Ethanol - E85 is 85% Ethanol mixed with 15% gasoline
Hydrogen - Combustion - like propane or Fuel-Cells to run electric motors.
Biodiesel - Vegetable Oil
See: Flex Fuels
Clean Fuels: An Overview
Alternative fuel vehicle at Wikipedia
Alternatives To Gasoline at Natural Resources of Canada
Bi-Fueled and Tri-Fueled Vehicles - How To Guides - DMV.ORG
Alternative Fuels Data Center at the DOE
Race Car fuels:
- NASCAR engines burn 110-octane leaded gasoline.
- Indy cars burn pure methanol (a.k.a. wood alcohol, CH3OH).
- Top Fuel dragsters and funny cars burn nitromethane (CH3NO2).
Hydrocarbon fuels come from a variety of sources. The primary one is refining of oil from under the ground.
Others included direct extraction of natural gas, production of alcohol from fermenting and other starch/sugar sources and biomass.
Refining:
Converting crude oil to different petrochemicals, chemical solvents and hydrocarbon chemicals is done by a process called refining which includes distillation and cracking.
Fractional distillation:
Oil is heated to boiling and the vapor formed is sent thru a long column containing trays which collect different fractions which condense at different temperatures. Products at these different ranges are frequently called "light naphtha" (C5-9), "heavy naphtha" (C7-9), "kerosene" (c10-16), "light gas oil", "heavy gas oil" (c16), and "reduced crude".
light gas oils, or fuel oils, can either be further split for manufacture of gasoline, or retained and refined for furnace oil or diesel fuel.
The lower boiling point hydrocarbon distillates are more valuable because they are major components of gasoline.
Kerosene and light gas oil fractions (also called middle distillates) are used in the production of kerosene, jet fuel, diesel fuel, and furnace oils.
The heavy gas oil may be used for heavy diesel fuel, industrial fuel oil
Vacuum distillation:
Larger-diameter columns are used to maintain comparable vapor velocities at reduced operating pressures. A vacuum of 50 to 100 millimeters of mercury absolute is produced by a vacuum pump or steam ejector.
The primary advantage of vacuum distillation is that it allows for distilling heavier materials at lower temperatures than those that would be required at atmospheric pressure, thus avoiding thermal cracking of the components.
Cracking:
Breaking larger molecules into smaller pieces.
Thermal and catalytic cracking produce the basic building blocks for petrochemicals butylene, ethylene, propylene. These basic products are processed further to give such materials as monopropylene glycol (MPG) and monoethylene glycol (MEG).
Hexadecane (C16H34), for example, can be split into various proportions of octane (C8H18), hexane (C6H12) and a small amount of ethylene (C2H4).
European refineries typically break a barrel of crude oil down into about 25% gasoline, 50% light gas oil/diesel where USA refineries typically break a barrel of crude down into around 50% gasoline and 25% diesel/heating oil.
A barrel of crude ultimately yields about 2% lubricating oils.
See:
How Oil Refining Works
Fractional Distillation at alcohols.co.uk
Fermentation:
Alcohol's are created by fermentation, a natural microbiological process where sugars are converted to alcohol by yeast (Saccharomyces cerevisiae - a type of fungi).
A small amount of nitrogen is added to a sugar such as molasses; the action of the yeast produces alcohol and carbon dioxide in 24 to 36 hours.
The reactions within the yeast
to make this happen are very complex but the overall process is as follows:
C6H12O6
====> 2(CH3CH2OH)
+ 2(CO2)
Sugar ====>
Alcohol
+ Carbon dioxide gas
(Glucose)
(Ethyl alcohol)
The resulting solution is distilled to get pure ethanol.
Source: http://www.yobrew.co.uk/fermentation.php
For each pound of simple sugars, yeast can produce
approximately 1/2 pound (0.15 gallons)
of ethanol and an equivalent amount of
carbon dioxide.
corn is used for ethanol production because of
the large amount of carbohydrates (Hydrated carbon) (84.1%), which consists of
Starch 72.0%, Fiber (NDF) 9.5% and Simple Sugars 2.6%.
Starch is processed by adding an enzyme (glucoamylase) which breaks down the starch into simple sugar (glucose) so it can be fermented with yeast.
C6H10O5 + H2O --> C6H12O6
Byproducts
include high fructose corn syrup (HFCS), biodegradable
plastics, food additives such as citric acid and xanthan
gum, corn oil (cooking oil), and livestock feed.
See: How Fuel ethanol is Made from Corn at ces.purdue.edu
What is the chemical nature of sugar? at answers.yahoo.com
Cellulosic ethanol also know by the name Ceetol, is a biofuel produced from wood, grasses, or the non-edible parts of plants.
Cellulose, the principal component of most plant cell wall and has the same basic chemical formula (C6H10O5) as starch,
but the molecules are held together with a different type of glycosidic bond.
It is converted to ethanol by using acids or enzymes to create sugars that are fermented.
Cellulosic ethanol can also be created by a thermo-chemical process, which uses various combinations of temperature, pressure, residence time, water, oxygen or air, and catalysts to convert biomass to cellulosic ethanol.
See: Producing Cellulosic Ethanol: Sugar Platform Vs. Thermo-Chemical
Biomass:
Methane can be produced/captured from animal manure, wastewater and
landfill gas.
See Biomass in Renewable Energy
Flame temperatures for some common fuel gases
| Fuel Gas | Oxygen
°C | Air
°C |
| Acetylene | 3,100 | 2,400 |
| Butane | | 1,970 |
| Ethane | | 1,960 |
| Hydrogen | 2,660 | 2,045 |
| MAPP1) | 2,980 | |
| Methane | 2,810 | 1,957 |
| Natural Gas | 2,770 | |
| Propane | 2,820 | 1,980 |
| Propane Butane Mix | | 1,970 |
| Propylene | 2,870 | |
Source: EngineeringToolBox.com
Other Terms:
- Alkanes (paraffins) - A family of hydrocarbons with the chemical formula CnH2n + 2. can be arranged either in straight chains (normal paraffins, such as propane, ethane, butane) or branched chains (isoparaffins).
The high-molecular mass (greater than c12 alkanes have a crystalline structure with with a solid waxy texture.
- Alkenes (olefins) Have carbon=carbon double bonds. e.g. 2-methyl-2-butene (C5H10)
- ALIPHATIC COMPOUNDS are composed of carbon atoms linked together in an open chain structure.
- ALICYCLIC COMPOUNDS are compounds in which the carbon atoms are arranged in a ring structure.
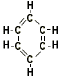 Aromatic Compounds are compounds which have structures related to that of benzene, the simplest aromatic compound. examples: Benzene, Toluene, ethyl benzene. Aromatic Compounds are compounds which have structures related to that of benzene, the simplest aromatic compound. examples: Benzene, Toluene, ethyl benzene.
- Benzene C6H6 - A compound with 6 carbon atoms in a ring as opposed to Hexane which is a chain. Not a good fuel. May be carcinogenic. Auto gas contains some of these.
- Chemical Hazards Response Information System (CHRIS)
- CNG - Compressed natural gas
- Cyclo-compound - Molecules which for a closed loop rather than a chain.
Saturated cyclo-compounds are called naphthenes.
- ETBE - Ethyl Tertiary Butyl Ether - An oxygenated compounds added to gasoline to reduce vehicle exhaust emissions.
- Ether is a class of organic compounds which contain an ether group -- an oxygen atom connected to two (substituted) alkyl or aryl groups.
Diethyl ether - A solvent and anesthetic (ethoxyethane, CH3-CH2-O-CH2-CH3)
See: Ether at ChemistryDaily.com
- characterized by a carbonyl group (O=C) linked to two other carbon atoms
- Flash Point - Lowest temperature at which the vapors can form an ignitable mixture in air.... but that just means BURN, not EXPLODE.
| Flash point |
| Fuel | °F |
| Gasoline | -40 |
| Coleman Fuel | -27 |
| Ethyl Alcohol | 55 |
| Solvent Naphtha | 60-75 |
| Heavy Naphtha | 95-105 |
| Kerosene | 100-160 |
| Naphthalene | 174 |
See: Flash Point - Fuels at engineeringtoolbox.com/
- Keytones - A functional group of organic compounds which contain a carbonyl group (O=C) linked to two other carbon atoms or a chemical compound that contains a carbonyl group.
Acetone CH3 CO CH3 is the simplest keytone. - A polar organic solvent and therefore dissolves a wide variety of substances. It has low chemical reactivity.
See: Ketone at ChemistryDaily.com
- LPG - Liquified Petroleum Gas - mixture of propane (90%) and other gases.
- LNG - Liquified Natural Gas (primarily methane, CH4)
- Material Safety Data Sheet (MSDS) MSDSsearch.com, ZenStoves.net/Fuels.htm, Hazard Communication at OSHA
- MTBE - Methyl Tertiary Butyl Ether - C5H12O. A gasoline additive, used as an oxygenate and to raise the octane number.Oxygen helps gasoline burn more completely, reducing harmful tailpipe emissions from motor vehicles.
- Naphtha (Stoddard solvent)- Refined or partly refined light distillates with an approximate boiling point range of 27 degrees to 221 degrees Centigrade. Blended further or mixed with other materials, they make high-grade motor gasoline or jet fuel. Also, used as solvents, petrochemical feed-stocks, or as raw materials for the production of town gas.
Usually contains:
[paraffins (C4 to C13), olefins naphthenes and a small amount of aromatic hydrocarbons. (e.g. benzene (C6H6),]. Used in making gasoline.
Created during the refining process of crude oil.
See: Naphtha at wikipedia and
Pocket Guide to Chemical Hazards at cdc.gov/niosh
- Naphthene - (IUPAC Name naphthalene)
Hydrocarbons which have one or more rings of carbon atoms. e.g. cycloopropane, cyclobutane (C4H8), ...
- Olefin - An unsaturated hydrocarbon containing at least one carbon-to-carbon double bond. THe simplest one is ethylene (C6H4)
- oxygenated motor gasoline - fuels blended with an additive, usually methyl tertiary butyl ether (MTBE) or ethanol to increase oxygen content, allowing more thorough combustion for reduced carbon monoxide emissions.
- Paraffins - Paraffins are an obsolescent term for alkanes. Still widely used in the petrochemical industry.
- Rubbing alcohol - Isopropyl or ethyl alcohol to which some methyl or acetone has been added to make it poisonous.
- SBP - Special Boiling Points
- TAME - Tertiary amyl methyl ether
- Wood Alcohol - Methanol alcohol
See Glossaries at:
Energy Information Agency (EIA) (www.eia.doe.gov/glossary/)
www.ConsumerEnergyCenter.org
The Institute for the Analysis of Global Security (iags.org).
Links:
Common Household product chemical composition
Fuel Chemistry
International Fuel Names
gasoline FAQ at faqs.org
A guide to Hydrocarbon fuels at the AMC
Hydrocarbons at Third Millennium Online
Hydrogen - Fuels
Hydrocarbons at Third Millennium Online
Fuel Chemistry
International Fuel Names
Boiling Points, Vapor Pressures, Melting Points, Flash Points
petroleum refining at Encyclopedia Britanica
Exhaust Gas Analysis
Hydrogen as a fuel - FAQ
An Introduction to Organic Chemistry
Home Heating Costs
Firewood
Fuels at the Camp Stove Page
Octane
Dry-cleaning solvents
The Energy Story - Chapter 8: Fossil Fuels - Coal, Oil and Natural Gas
Return to Chemistry
last updated 14 Nov 2008
|
